Lithium polymer batteries, commonly known as LiPo batteries, have revolutionized portable power with their lightweight design, high energy density, and flexible form factors. In this comprehensive guide, we delve into the intricacies of lithium polymer batteries, exploring their definition, basic structure, key materials, the rationale behind material choices, and how variations in these materials influence battery performance.
What Are Lithium Polymer Batteries?
A LiPo battery is a type of rechargeable battery that uses a polymer electrolyte instead of a liquid one. Unlike traditional lithium-ion batteries that use a liquid or gel electrolyte, LiPo batteries employ a solid or semi-solid polymer composite that acts as both the electrolyte and separator. This innovation results in a lighter and more adaptable battery design, making them ideal for consumer electronics, drones, electric vehicles, and various portable devices.
Studies have shown that LiPo batteries offer improved safety and a higher energy-to-weight ratio compared to conventional batteries (Battery University). Their design flexibility allows manufacturers to create custom shapes and sizes, which is a major advantage in modern product design.
Basic Structure of a LiPo Batteries
At its core, a lithium polymer battery consists of several key components:
- Cathode (Positive Electrode): Usually made of a lithium metal oxide (such as LiCoO₂, LiMn₂O₄, or LiFePO₄) that serves as the source of lithium ions during discharge.
- Anode (Negative Electrode): Typically composed of graphite or other carbon-based materials, which act as the host for lithium ions during the charge cycle.
- Polymer Electrolyte: A solid or gel-like substance that conducts lithium ions between the electrodes. It also serves as a separator, preventing direct contact between the cathode and anode.
- Current Collectors: Thin sheets of metal (often aluminum for the cathode and copper for the anode) that facilitate electron flow.
- Packaging: The outer casing, which is designed to protect the internal components while being as light and thin as possible.
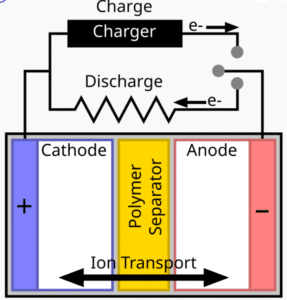
Each of these components plays a critical role in the overall performance, safety, and longevity of the battery. The integration of the polymer electrolyte enables a reduction in leakage risks and improves overall battery stability (Science Direct).
Materials Used in a LiPo Batteries
The performance of a LiPo battery is intrinsically linked to the materials used in its construction. Here, we detail the primary materials that make up these batteries:
Cathode Materials
The cathode in a LiPo battery is usually composed of lithium metal oxides. The most common materials include:
- Lithium Cobalt Oxide (LiCoO₂): Known for its high energy density, though it can be expensive and less stable at higher temperatures.
- Lithium Manganese Oxide (LiMn₂O₄): Offers a good balance between cost and performance.
- Lithium Iron Phosphate (LiFePO₄): Favoured for its stability, safety, and long cycle life, albeit with a slightly lower energy density.
Anode Materials
Graphite is the predominant material used for the anode due to its ability to reversibly intercalate lithium ions. However, research is ongoing into alternative materials such as:
- Silicon-based composites: Which can potentially increase capacity, though challenges remain with volumetric expansion during charge/discharge cycles.
Polymer Electrolytes
Polymer electrolytes are crucial for the LiPo battery’s flexibility and safety. Commonly used polymers include:
- Polyethylene Oxide (PEO): Often combined with lithium salts to facilitate ionic conductivity.
- Polyacrylonitrile (PAN): Offers good mechanical strength and stability.
- Poly(vinylidene fluoride) (PVDF): Known for its chemical resistance and high dielectric constant, which improves the ion transport mechanism.
Current Collectors and Additives
- Aluminum and Copper Foils: Serve as current collectors, ensuring efficient electron flow.
- Binders and Conductive Additives: Such as polyvinylidene fluoride (PVDF) binder and carbon black are used to enhance the structural integrity and electrical connectivity of the electrodes.
These materials are selected not only for their intrinsic properties but also for how well they integrate into the battery’s overall design, balancing performance, cost, and safety.
Why Choose These Materials?
The selection of materials in a LiPo battery is driven by several critical factors:
High Energy Density
Materials such as lithium cobalt oxide are favored for their high energy density, which allows for longer-lasting power in compact sizes. This is particularly important for consumer electronics where space and weight are at a premium.
Safety and Stability
The use of a polymer electrolyte significantly enhances safety. Unlike liquid electrolytes, polymer-based electrolytes reduce the risk of leakage and thermal runaway. This choice is backed by extensive research which has shown that solid or gel-like electrolytes contribute to a more robust battery design.
Mechanical Flexibility
The incorporation of polymers not only improves safety but also enables flexible battery designs. This is crucial for modern devices such as smartphones, wearables, and flexible electronics, where the battery may need to conform to irregular shapes.
Cost-Effectiveness and Scalability
Materials such as graphite and common lithium metal oxides are widely available and relatively cost-effective. Their scalability makes them ideal for mass production without compromising on quality. Furthermore, ongoing research into alternative materials like silicon composites is aimed at achieving even higher capacities while keeping costs manageable.
Environmental Impact
Choosing materials with a lower environmental footprint is increasingly important. Advances in battery technology are not only focused on performance but also on reducing environmental impact through sustainable sourcing and recycling practices.
The combination of these factors ensures that the LiPo battery remains a competitive choice for a wide range of applications, balancing performance with safety and cost.
How Material Quantities Affect Battery Performance
The quantity and quality of each material used in a lithium polymer battery play a crucial role in determining the battery’s overall performance. Small changes in material loading or composition can have significant implications for key parameters such as capacity, voltage, current delivery, and charge/discharge efficiency. In this expanded discussion, we delve deeper into the various ways material quantities influence battery performance.
Capacity
Cathode and Anode Loading:
- Cathode Material Loading: Increasing the active material (e.g., LiCoO₂, LiMn₂O₄, or LiFePO₄) directly boosts the battery’s energy storage capacity. However, there is a balance to be struck. Overloading the cathode can lead to a thicker electrode layer, which may hinder the diffusion of lithium ions, leading to higher internal resistance and lower power output.
- Anode Material Optimization: Similarly, optimizing the amount of anode material (commonly graphite) is essential. Adequate anode material ensures that there is sufficient host structure for lithium ions during charging. An imbalance between the cathode and anode capacities can lead to underutilization of the cell’s full potential or even accelerated degradation over time.
Voltage and Current Delivery
Electrode Thickness and Material Distribution:
- Electrode Thickness: The thickness of both the cathode and anode layers influences how quickly lithium ions can migrate through the electrode material. Thicker electrodes can store more charge, which is beneficial for capacity, but they also introduce longer ion transport paths. This can limit the maximum current the battery can deliver, particularly during high-load conditions.
- Uniform Material Distribution: A uniform distribution of active materials is critical. Inhomogeneities in material quantities can create localized areas of high resistance, affecting the overall voltage stability and reducing the effective current delivery. This uniformity is also vital to avoid hot spots during discharge, which can impair performance.
Charge/Discharge Efficiency and Cycle Life
Role of Binders, Additives, and Electrolyte Concentration:
- Binders and Conductive Additives: Binders like polyvinylidene fluoride (PVDF) and conductive additives such as carbon black are essential to maintain the structural integrity of the electrode. The quantity of these additives must be optimized; too much binder can reduce the electrode’s electrical conductivity, while insufficient binder may lead to mechanical degradation and premature capacity loss during cycling.
- Electrolyte Concentration: The concentration of lithium salts within the polymer electrolyte determines the ionic conductivity. An optimal concentration facilitates rapid ion transport during charging and discharging. However, if the electrolyte is too dilute, ionic mobility decreases; if it is too concentrated, the viscosity increases, both of which can negatively impact charge/discharge rates and efficiency.
Thermal Management and Safety
Heat Dissipation and Thermal Stability:
- Material Thermal Properties: The selected materials and their quantities also determine the battery’s thermal characteristics. Adequate amounts of heat-dissipating materials help maintain a uniform temperature during high-current operations, reducing the risk of overheating and thermal runaway.
- Electrolyte and Additive Balance: The formulation of the polymer electrolyte, combined with proper conductive additives, is crucial for managing the internal temperature. A well-optimized mix allows for efficient heat distribution and minimizes the development of thermal gradients across the battery cell.
An imbalance in these materials can lead to inefficient thermal management, potentially shortening the battery’s lifespan and posing safety risks during operation.
Integrated Effects on Overall Performance
Synergistic Optimization:
The performance of a LiPo battery does not depend on any single material parameter but on the synergy between various components. For example:
- A higher cathode loading can improve capacity, but only if the anode can accommodate the corresponding amount of lithium ions.
- Increasing electrode thickness might boost capacity, yet without a matching improvement in ion conductivity (via electrolyte optimization), the battery might suffer from slow charge/discharge rates.
- Thermal stability, charge efficiency, and cycle life are all interrelated, meaning that adjustments in one component necessitate compensatory changes in others to maintain overall balance.
Conclusion
In summary, a LiPo battery is much more than a simple power source. Its advanced design, incorporating state-of-the-art materials like lithium metal oxides, graphite, and polymer electrolytes, allows it to deliver high energy density, exceptional safety, and remarkable design flexibility. The precise formulation of these materials is critical; even minor adjustments in their quantities can significantly affect the battery’s capacity, voltage, current, and overall performance.
Understanding these intricate details helps manufacturers optimize battery performance and informs consumers about the technological innovations behind their devices. As research and development continue to evolve, we can expect further enhancements in lithium polymer batteries that will drive the next generation of portable power solutions.
By exploring the materials and design intricacies behind LiPo batteries, this article aims to provide valuable insights for both industry professionals and tech enthusiasts. The ongoing evolution in battery materials and technology is paving the way for safer, more efficient, and more adaptable energy storage solutions in our ever-changing technological landscape.
Feel free to share this comprehensive guide to help others understand what makes a lithium polymer battery truly exceptional.