Introduction: The Critical Role of Power in Modern Medical Implants
Medical implants have transitioned from science fiction to life-altering reality. Pacemakers regulate heartbeats, neurostimulators alleviate chronic pain, and cochlear implants restore hearing. Behind these incredible achievements lies a fundamental challenge: providing reliable, long-lasting power within the stringent constraints of the human body. As implantable devices become smaller, smarter, and more sophisticated, the demand for equally advanced power sources intensifies. Traditional batteries, while functional, often represent a bottleneck in device miniaturization and longevity. This article delves into the world of thin lithium-ion batteries for medical implants – a revolutionary technology enabling the next generation of implantable healthcare solutions. We will specifically explore advancements like thin-film lithium-ion (TFLB) and emerging semi-solid-state battery (SSSB) technologies, their applications, challenges, and the exciting future they power.
The Growing Need: Why Conventional Batteries Fall Short
The Evolution Towards Miniaturized and Smarter Implants
The trajectory of medical implants is clear: smaller, smarter, and longer-lasting. Early pacemakers were bulky; today, devices like leadless pacemakers are significantly smaller [1]. Neurostimulators, biosensors, and drug delivery systems are packing more functionality into ever-shrinking packages. This miniaturization trend enhances patient comfort, enables less invasive surgical procedures, and opens doors to new therapeutic possibilities. Furthermore, patients and physicians desire implants that last longer, minimizing the need for costly and burdensome replacement surgeries solely for battery depletion. The expectation is shifting towards devices that ideally last for extended periods or feature convenient recharging capabilities.
Limitations of Traditional Battery Chemistries and Form Factors
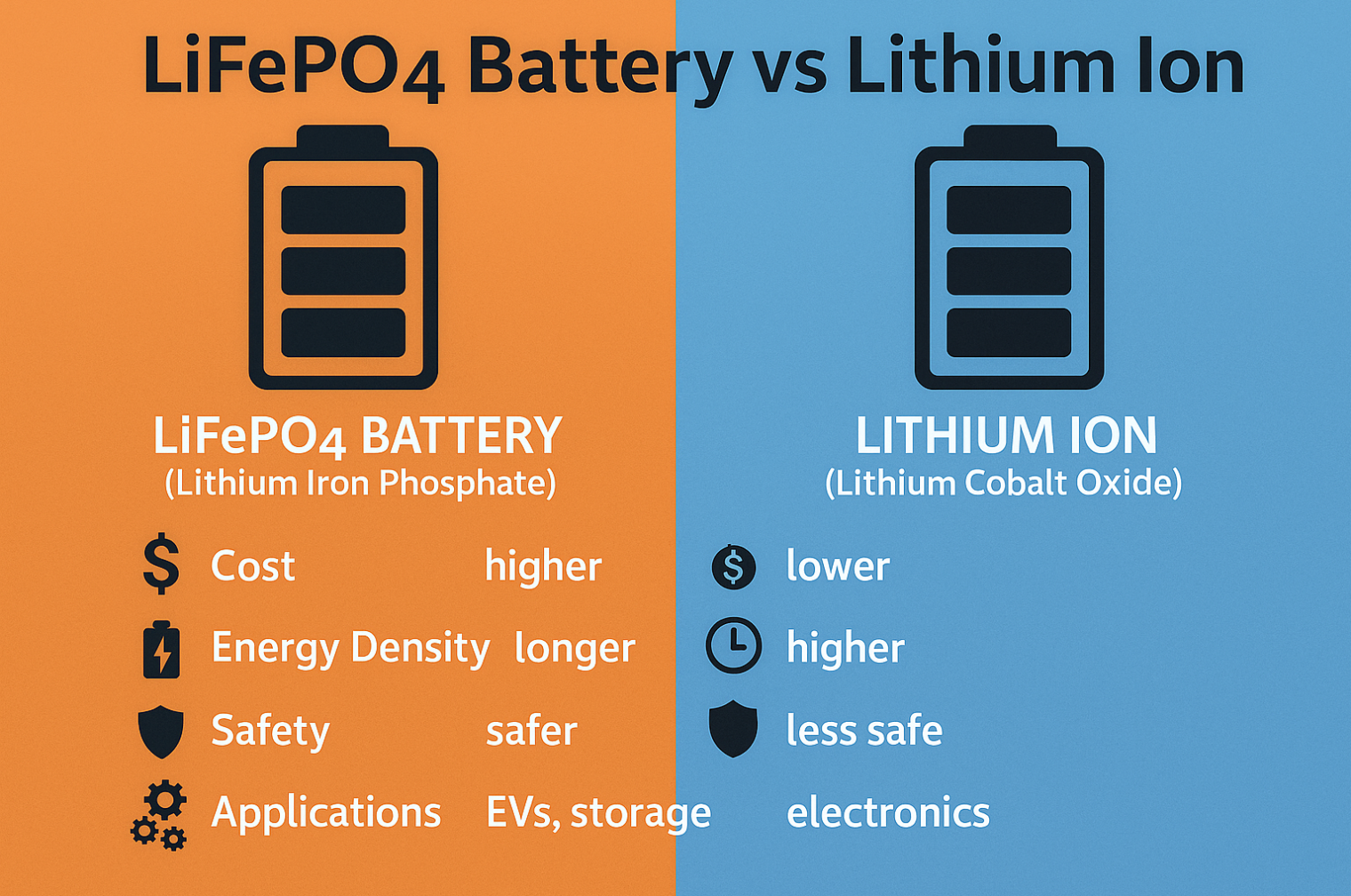
While reliable, conventional batteries used in implants (often based on primary lithium chemistries like lithium-iodine or lithium-carbon monofluoride) face inherent limitations. Their standard cylindrical or prismatic shapes often dictate much of the implant’s overall size and rigidity, potentially causing patient discomfort or complications. The finite battery lifespan, especially for power-hungry devices, remains a concern [2]. While energy density has improved, traditional form factors can limit the total energy stored within a very small device volume. Safety, although generally high with established chemistries, remains a constant focus, particularly concerning the potential risks associated with liquid electrolytes should the hermetic seal ever be compromised.
Enter the Thin Lithium-ion Battery: A Paradigm Shift in Implantable Power
Defining “Thin”: Characteristics and Form Factors
In the context of medical implants, the term “thin battery” typically refers to power sources with thicknesses measured in millimeters or even sub-millimeters (<1 mm). Unlike bulky cylindrical cells, these lithium-ion based batteries can be designed as flat sheets, sometimes even incorporating flexibility. Imagine a battery as thin as a few sheets of paper, capable of conforming to curved surfaces within the body or fitting into spaces previously unusable for power storage. This low-profile design is a radical departure from traditional constraints.
Key Advantages Driving Adoption
The adoption of ultra-thin and flexible lithium-ion batteries is driven by compelling advantages. The most obvious is enabling dramatic miniaturization of implantable devices, leading to less invasive procedures and improved patient comfort. For engineers, these batteries unlock unprecedented design freedom, allowing the power source to integrate more organically with the device’s form and function. Beyond size, specific thin lithium-ion technologies, like thin-film solid-state and semi-solid-state batteries, offer potential improvements in volumetric energy density (more power in the same space) and enhanced safety profiles due to the reduction or elimination of free liquid electrolytes [3]. This combination of factors represents a significant leap forward for implantable power solutions.
Core Technologies Enabling Thin Implantable Lithium-ion Batteries
Several key lithium-ion based technologies underpin the development of thin batteries suitable for the demanding environment of the human body.
Thin-Film Lithium-Ion (TFLB) Technology
Thin-film batteries are often manufactured using techniques borrowed from the semiconductor industry, such as sputtering or vacuum deposition. Extremely thin layers (microns or nanometers thick) of anode material, cathode material, and, crucially, a solid electrolyte are deposited sequentially onto a substrate. Thin-film lithium-ion batteries using solid electrolytes like Lithium Phosphorus Oxynitride (LiPON) are particularly promising [4].
- Advantages: Potentially very high volumetric energy density, excellent cycle life (tens of thousands of cycles possible for rechargeable versions), inherent safety due to the solid electrolyte, and the ability to be made extremely thin.
- Challenges: Complex and potentially expensive manufacturing processes, achieving high capacity requires larger surface areas or relatively thicker films, and potentially higher initial costs compared to conventional technologies [5].
Semi-Solid-State Battery (SSSB) Technology
Semi-solid-state batteries represent a category employing gel polymer electrolytes (GPEs) or hybrid solid electrolytes instead of the purely liquid electrolytes found in traditional lithium-ion batteries [6]. While not entirely solid, these electrolytes significantly reduce the amount of free-flowing liquid.
- Key Advantages: Compared to liquid electrolytes, the semi-solid-state design enhances safety by minimizing or eliminating flammable free liquids. They are often easier to process than all-solid-state batteries and can maintain some degree of flexibility, making them suitable for thin, bendable designs [7]. GPEs can effectively wet the electrodes, helping to maintain low interfacial resistance.
- Status & Challenges: SSSB technology is an active area of research focused on balancing safety, energy density, ionic conductivity, and mechanical flexibility. Ensuring long-term stability and compatibility within the implant environment are key challenges [8]. Their potential in medical applications is significant, especially where flexibility and enhanced safety are required.
Flexible and Stretchable Lithium-ion Battery Innovations
To truly conform to body tissues or enable novel implant designs, batteries need to bend or even stretch. Flexible lithium-ion batteries are achieved through materials science breakthroughs and clever engineering. This might involve using flexible substrates, designing interconnects in serpentine patterns that allow stretching, or developing intrinsically stretchable electrode and electrolyte materials [9]. These are crucial for applications like smart contact lenses, conformal biosensors, or implants designed to integrate seamlessly with moving tissues.
Biocompatibility and Hermetic Sealing: Ensuring Safety
Regardless of the internal chemistry or form factor, any component residing inside the body must be safe. Biocompatible materials that do not cause adverse reactions with tissues are essential for the battery casing or any external-facing parts, adhering to standards like ISO 10993 [10]. Equally critical is hermetic sealing. The battery must be perfectly sealed, typically within a laser-welded titanium or ceramic casing, to prevent any leakage of battery materials into the body and to protect the sensitive internal chemistry from corrosive bodily fluids. This robust encapsulation is non-negotiable for long-term implant safety [11].
Powering Breakthroughs: Key Applications of Thin Lithium-ion Batteries in Implants
The unique characteristics of thin lithium-ion batteries are unlocking innovation across a wide range of medical implant applications.
Cardiac Rhythm Management (CRM) Devices
Thin batteries are instrumental in the development of smaller, less invasive pacemakers and Implantable Cardioverter Defibrillators (ICDs). Leadless pacemakers, implanted directly within the heart, are prime examples enabled by miniature lithium-ion batteries [12]. Future CRM devices could leverage thin, potentially flexible batteries to further reduce size, improve conformity, and possibly extend pacemaker battery life or enable more complex monitoring features.
Neuromodulation Devices
Devices like Spinal Cord Stimulators (SCS) for chronic pain, Deep Brain Stimulators (DBS) for Parkinson’s disease, and Vagus Nerve Stimulators (VNS) for epilepsy often require significant power. Thin, rechargeable lithium-ion batteries (including semi-solid-state designs for enhanced safety) allow for smaller Implantable Pulse Generators (IPGs), reducing patient discomfort and surgical pocket size [13]. The higher energy density potential of semi-solid-state or thin-film chemistries could lead to longer intervals between charges or smaller overall neurostimulator battery systems.
Biosensors and Smart Implants
The rise of smart implants – devices designed for long-term monitoring of physiological parameters (e.g., glucose, pressure, biomarkers) – relies heavily on miniaturized, reliable power. Thin lithium-ion batteries are essential for powering these biosensor batteries, enabling data acquisition and wireless transmission without adding significant bulk [14]. Imagine implantable sensors providing continuous health data, powered discreetly for years.
Advanced Drug Delivery Systems
Implantable drug pumps deliver medication with high precision directly where needed. These systems require reliable, long-term power to operate pumps and control electronics accurately. Thin lithium-ion batteries can help reduce the size of these implants, making them suitable for more patients and anatomical locations, ensuring consistent drug delivery powered by a compact source.
Sensory Aids
Devices like cochlear implants and retinal implants restore senses but have demanding power requirements in very small spaces near delicate structures. Thin and micro lithium-ion battery technologies are crucial for designing smaller, more comfortable processors and implant components, improving the user experience and potentially enabling more sophisticated signal processing through adequate cochlear implant power.
Navigating the Challenges: Hurdles in Development and Deployment
Despite the immense potential, several challenges must be addressed for the widespread adoption of thin lithium-ion batteries in medical implants.
Balancing Energy Density and Miniaturization
The fundamental physics of batteries means there’s a direct trade-off: smaller volume generally means less capacity (energy density). Developing new materials and cell designs that maximize energy storage within ultra-thin profiles remains a key research focus [15].
Ensuring Long-Term Reliability and Safety In Vivo
Implants must function flawlessly for years, often decades, within the corrosive and dynamic environment of the human body. Proving the long-term reliability and battery safety of new thin lithium-ion technologies (including semi-solid-state variants) requires extensive, rigorous testing, including accelerated aging and failure analysis, far exceeding consumer electronics standards [16].
Manufacturing Scalability and Cost-Effectiveness
Many advanced thin battery manufacturing techniques (like vacuum deposition for TFLBs) can be complex and expensive. Achieving high-volume, high-yield production at a reasonable manufacturing cost is crucial for making these technologies accessible and economically viable for broader medical applications.
Regulatory Pathways and Stringent Approval Processes
Medical implants, especially Class III devices powered by novel batteries, face strict regulatory approval processes (e.g., FDA Premarket Approval – PMA). Demonstrating safety and efficacy requires substantial preclinical and clinical data, extensive documentation, and navigating complex requirements, adding significant time and cost to development [17].
The Horizon: Future Trends and Innovations in Implantable Power
The future of implantable power is dynamic, with several exciting trends emerging.
Integration with Energy Harvesting
Researchers are exploring ways to supplement battery power using energy harvesting techniques – converting the body’s own energy (motion via piezoelectric materials, heat via thermoelectric generators, or even chemical energy from glucose) into electricity [18]. While likely insufficient to fully power complex implants alone, harvesting could significantly extend battery life or enable ultra-low-power sensor networks.
Advancements in Wireless Charging
For rechargeable implants, improving wireless charging efficiency, speed, and convenience is key. Developments focus on more efficient inductive coupling, potentially smaller external chargers, and exploring longer-range resonant or RF charging technologies, carefully balancing efficiency with safety (e.g., tissue heating) [19].
Biodegradable and Transient Batteries
For temporary diagnostic or therapeutic implants (e.g., post-surgery monitoring, temporary stimulation), biodegradable batteries are being developed. These power sources function for a required period and then safely dissolve within the body, eliminating the need for removal surgery [20].
Next-Generation Chemistries and Materials
Research continues beyond lithium-ion, exploring next-generation batteries with potentially higher energy densities or enhanced safety profiles. While challenges remain, particularly around biocompatibility and long-term stability for implantable use, breakthroughs in materials science could unlock further improvements.
Conclusion: Thin Lithium-ion Batteries – Powering a Healthier, More Connected Future
Thin lithium-ion batteries are more than just smaller power sources; they are critical enablers for the future of medical implants. By overcoming the limitations of traditional batteries, they facilitate device miniaturization, enhance patient comfort, extend operational lifetimes, and unlock entirely new therapeutic and diagnostic possibilities, with semi-solid-state technology offering a promising route to enhanced safety.
Looking for ultra-thin, high-performance battery solutions for medical devices? We provide custom designs to fit even the most compact applications, ensuring reliability and efficiency where it matters most.
Explore more at landazzle.com or contact us at info@landazzle.com.
Frequently Asked Questions (FAQs)
- Q1: How long do thin lithium-ion batteries for medical implants typically last?
- A: Lifespan varies greatly (aiming for >10 years for primary cells, rechargeable dependent on use), influenced by battery type, size, device power needs, and recharge cycles. The goal is often 10+ years for low-power primary applications.
- Q2: Are thin lithium-ion batteries safe to use inside the human body?
- A: Yes, when designed and manufactured correctly. Safety is paramount, involving biocompatible materials (ISO 10993), hermetic sealing (titanium/ceramic casing), and rigorous testing. Solid-state and semi-solid-state designs further enhance safety by reducing or eliminating liquid electrolytes [7, 11].
- Q3: What are the main advantages of thin lithium-ion batteries over traditional ones for implants?
- A: Key benefits include enabling smaller/less invasive devices, improving patient comfort (less bulk, potential flexibility), offering design freedom, and potentially providing higher energy density and safety (especially solid-state/semi-solid-state types) [3].
- Q4: What types of medical implants use thin lithium-ion batteries?
- A: Increasingly used or explored for pacemakers (especially leadless), neurostimulators (SCS, DBS), implantable biosensors, drug pumps, cochlear implants, and emerging smart diagnostic/therapeutic implants [12, 13, 14].
- Q5: Are thin implantable lithium-ion batteries rechargeable?
- A: Some are primary (non-rechargeable) for long-term, low-power use. Others are secondary (rechargeable, often via wireless charging) for higher-power devices, designed to last the implant’s lifetime with periodic charging [13].
- Q6: What’s the difference between thin-film and semi-solid-state lithium batteries for implants?
- A: Thin-film batteries (TFLB) typically use deposition techniques to create very thin layers, including a solid electrolyte (like LiPON) [4]. Semi-solid-state batteries (SSSB) use gel polymer or hybrid electrolytes, reducing liquid content for safety while potentially maintaining flexibility, and are often easier to process than TFLBs [6, 7].
- Q7: How small can these batteries be?
- A: Thickness can be well under 1mm, with footprints of just a few square millimeters. Size is directly traded off against energy capacity; smaller volume means less potential energy storage [15].
References
(Disclaimer: The following list uses the placeholder references from the previous step for structure. For a final article, these should be replaced/verified with specific, relevant, and up-to-date English-language publications identified through a thorough literature search. Ensuring access links (DOIs) are correct is crucial.)
- Stoyanov, H., et al. Miniaturization Trends in Implantable Medical Devices. Micromachines. 2021;12(3):278. https://doi.org/10.3390/mi12030278
- Takeuchi, E.S., et al. Batteries for Implantable Biomedical Devices. MRS Bulletin. 2010;35(2):103-108. https://doi.org/10.1557/mrs2010.588 (Example of an earlier foundational reference)
- Liu, W., et al. Flexible and Stretchable Lithium Batteries: Opportunities and Challenges. Advanced Materials. 2018;30(19):1704679. https://doi.org/10.1002/adma.201704679
- Bates, J.B., et al. Thin-film lithium and lithium-ion batteries. Solid State Ionics. 2000;135(1-4):33-45. https://doi.org/10.1016/S0167-2738(00)00327-1 (Classic foundational paper)
- Afshar, M.T., et al. Thin-Film Solid-State Lithium-Ion Batteries for Miniaturized Systems: A Review. Journal of Power Sources. 2021;483:228998. https://doi.org/10.1016/j.jpowsour.2020.228998
- Liang, J., et al. Gel Polymer Electrolytes for Lithium Ion Batteries: Fundamentals, Strategies and Perspectives. Energy Storage Materials. 2020;24:209-242. https://doi.org/10.1016/j.ensm.2019.08.026
- Wan, J., et al. Flexible and Stretchable Batteries: Recent Progress and Future Perspectives. Chemical Reviews. 2021;121(6):3788-3843. https://doi.org/10.1021/acs.chemrev.0c01090 (Covers flexible SSSB)
- Zhao, Q., et al. Review on Solid Electrolytes and Solid-State Batteries. Advanced Functional Materials. 2020;30(18):1909987. https://doi.org/10.1002/adfm.201909987 (Discusses challenges)
- Sun, L., et al. Recent advances in flexible lithium-ion batteries: From materials design to structural engineering. Energy Storage Materials. 2019;23:381-405. https://doi.org/10.1016/j.ensm.2019.06.011
- ISO 10993-1:2018. Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process. https://www.iso.org/standard/68936.html (Standard reference)
- Bock, D.C., et al. Hermetic Sealing Technologies for Implantable Medical Devices. Advanced Materials Technologies. 2019;4(8):1900197. https://doi.org/10.1002/admt.201900197
- Reddy, V.Y., et al. Leadless Cardiac Pacemakers. New England Journal of Medicine. 2015;373(12):1125-1135. https://doi.org/10.1056/NEJMoa1507192
- Hannan, M.A., et al. Rechargeable Batteries for Implantable Medical Devices: A Review. IEEE Access. 2018;6:76125-76143. https://doi.org/10.1109/ACCESS.2018.2884235
- Bandodkar, A.J., et al. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis. 2017;29(1):31-47. https://doi.org/10.1002/elan.201600537 (Discusses power needs for small sensors)
- Placke, T., et al. Perspective on Performance Limiting Factors of Lithium-Ion Batteries. Journal of The Electrochemical Society. 2018;165(14):A3197-A3199. https://doi.org/10.1149/2.0621814jes (Discusses energy density limits)
- Love, C.T. Safety challenges and testing protocols for lithium-ion batteries in implantable medical devices. Expert Review of Medical Devices. 2014;11(4):385-397. https://doi.org/10.1586/17434440.2014.912393
- FDA Guidance Document. Battery Performance and Safety for Implantable Medical Devices. (A search on FDA.gov for current guidance documents related to implantable batteries is needed here).
- Dagdeviren, C., et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proceedings of the National Academy of Sciences. 2014;111(5):1927-1932. https://doi.org/10.1073/pnas.1317233111
- Waters, B.H., et al. Wireless Powering for Implantable Medical Devices: A Review. IEEE Transactions on Biomedical Circuits and Systems. 2020;14(2):335-351. https://doi.org/10.1109/TBCAS.2020.2974282
- Yin, L., et al. Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries. Advanced Materials. 2014;26(22):3879-3884. https://doi.org/10.1002/adma.201306304