Electrolitos poliméricos sólidos y de gel: El futuro de la tecnología de las baterías
El mundo funciona con baterías. Desde los teléfonos inteligentes que llevamos en el bolsillo hasta los vehículos eléctricos que circulan por nuestras carreteras, la demanda de almacenamiento de energía fiable y de alto rendimiento es cada vez mayor. Sin embargo, las baterías tradicionales de iones de litio que alimentan nuestra vida moderna tienen limitaciones. Entre en electrolitos poliméricos sólidos y en geluna tecnología revolucionaria que está a punto de dar paso a la próxima generación de baterías más seguras, potentes y duraderas.
Durante décadas, las baterías de iones de litio han utilizado electrolitos líquidos para transportar iones entre el ánodo y el cátodo. Aunque eficaces, estas soluciones líquidas conllevan riesgos inherentes. Suelen ser inflamables, pueden tener fugas si la batería se daña y contribuyen a la formación de dendritas, pequeñas estructuras en forma de aguja que pueden provocar cortocircuitos y fallos en la batería. Estos problemas de seguridad se han hecho más acuciantes a medida que demandamos más energía de baterías más pequeñas y ligeras.
Los electrolitos poliméricos sólidos y en gel están llamados a revolucionar el sector. Al sustituir el líquido volátil por un material polimérico sólido o semisólido, podemos crear baterías que no sólo son fundamentalmente más seguras, sino que también tienen el potencial de una densidad de energía significativamente mayor. Este artículo explora todo lo que necesita saber sobre esta apasionante frontera en la tecnología de las baterías.
¿Qué son los electrolitos poliméricos sólidos y en gel?
En el fondo, un electrolito polimérico es un material que combina un polímero con una sal, normalmente de litio, para crear un medio de transporte de iones. El polímero aporta la integridad estructural, mientras que la sal proporciona los portadores de carga (iones). Actualmente se están desarrollando dos tipos principales de electrolitos poliméricos:
Componentes básicos: Anfitrión polimérico y sales de litio
La base de cualquier electrolito polimérico es el polímero huésped. Se trata de una molécula de cadena larga que forma una matriz capaz de disolver las sales de litio y facilitar el movimiento de los iones de litio. Entre los polímeros comunes utilizados en esta aplicación se incluyen:
- Poli(óxido de etileno) (PEO): Uno de los hospedadores poliméricos más estudiados debido a su excelente capacidad para disolver sales de litio.
- Poli(acrilonitrilo) (PAN): Conocido por su elevada constante dieléctrica y su buena estabilidad mecánica.
- Poli(fluoruro de vinilideno) (PVDF): A menudo se utiliza en electrolitos poliméricos en gel debido a su capacidad para atrapar electrolitos líquidos.
El otro componente crucial es el sal de litioque proporciona los iones de litio que viajan entre los electrodos. La elección de la sal afecta a la conductividad iónica del electrolito y a su estabilidad general.
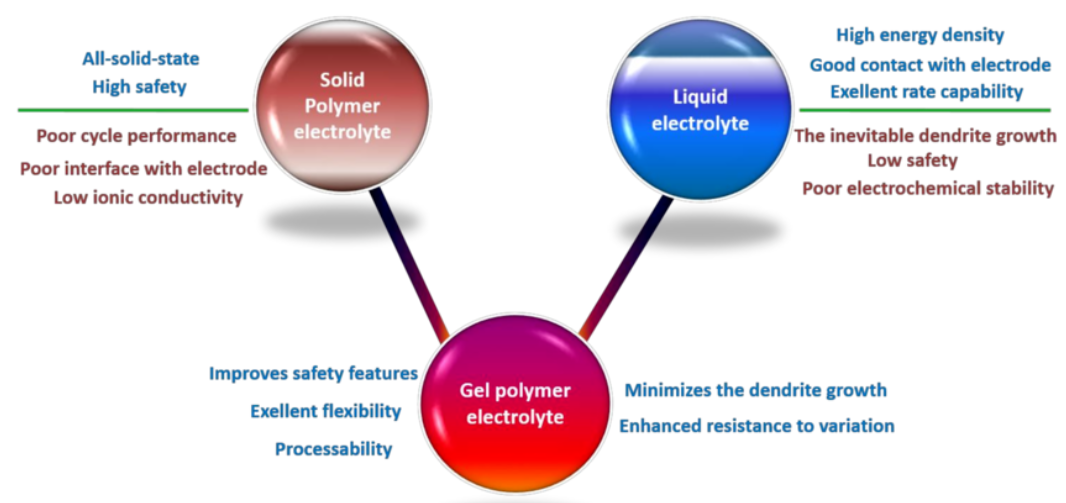
Electrolitos poliméricos sólidos (SPE): La verdadera visión del estado sólido
A electrolito polimérico sólido (SPE) es un material completamente sólido y sin disolventes. Se trata esencialmente de una película fina y flexible de polímero con sal de litio disuelta. El objetivo de las SPE es crear una batería verdaderamente sólida, sin ningún componente líquido.
La principal ventaja de las SPE es la seguridad. Al eliminar el electrolito líquido inflamable, se reduce drásticamente el riesgo de incendio de las baterías. Además, la naturaleza sólida del electrolito puede bloquear físicamente el crecimiento de dendritas, lo que podría permitir el uso de ánodos metálicos de litio de alta capacidad y aumentar significativamente la densidad energética.
Electrolitos poliméricos en gel (GPE): Un enfoque híbrido
A electrolito polimérico en gel (GPE)también conocido como electrolito semisólido, representa un compromiso entre los electrolitos sólidos y líquidos. En un GPE, un electrolito líquido queda atrapado en la estructura porosa de una matriz polimérica. Es como si una esponja (el polímero) absorbiera agua (el electrolito líquido).
Este enfoque híbrido ofrece varias ventajas. Los GPE presentan una conductividad iónica a temperatura ambiente en comparación con los SPE, lo que resulta crucial para una carga rápida y una elevada potencia de salida. También mantienen un mejor contacto con los electrodos que sus homólogos sólidos, lo que ayuda a reducir la resistencia interfacial. Aunque no son tan seguros como los SPE, los GPE suponen una mejora significativa respecto a los electrolitos líquidos tradicionales, ya que su consistencia gelatinosa evita las fugas.
La ciencia detrás del rendimiento: ¿Cómo funcionan?
El rendimiento de una batería depende en gran medida de las propiedades de su electrolito. En el caso de los electrolitos poliméricos sólidos y en gel, entran en juego algunos principios científicos clave.
Conductividad iónica: La clave de la potencia
Conductividad iónica es una medida de la capacidad de un electrolito para conducir iones. En una batería de iones de litio, una mayor conductividad iónica significa que los iones de litio pueden moverse más rápidamente entre el ánodo y el cátodo. Esto se traduce en tiempos de carga más rápidos y una mayor capacidad de suministrar energía para aplicaciones exigentes.
Los electrolitos líquidos tradicionales tienen una excelente conductividad iónica, razón por la que han sido la norma durante tanto tiempo. El reto de los electrolitos poliméricos sólidos ha sido conseguir niveles comparables de conductividad, especialmente a temperatura ambiente. Los electrolitos poliméricos en gel salvan esta distancia al incorporar un componente líquido que mejora notablemente el transporte de iones.
El papel de la matriz polimérica en el transporte de iones
Tanto en los electrolitos poliméricos sólidos como en los de gel, la matriz polimérica desempeña un papel crucial en el movimiento de los iones. Las cadenas poliméricas contienen átomos como el oxígeno que pueden enlazarse débilmente con los iones de litio. Cuando las cadenas poliméricas se mueven y flexionan, ayudan a transportar los iones de litio, creando una vía de conducción. La flexibilidad de estas cadenas poliméricas es un factor clave para determinar la conductividad iónica del electrolito.
Estabilidad interfacial: Prevención de la degradación y las dendritas
La interfaz entre el electrolito y los electrodos es una zona crítica en una batería. Una mala estabilidad interfacial puede dar lugar a multitud de problemas, entre ellos la temida formación de dendritas. En las baterías con electrolitos líquidos, los iones de litio pueden depositarse de forma irregular en el ánodo durante la carga, formando estructuras afiladas en forma de aguja. Estas dendritas pueden atravesar el separador y provocar un cortocircuito en la batería, con el consiguiente riesgo de fuga térmica e incendio.
Los electrolitos poliméricos sólidos son especialmente prometedores en este sentido porque su estructura rígida puede actuar como barrera física al crecimiento de dendritas. Esta mayor estabilidad podría liberar por fin el potencial de los ánodos metálicos de litio, que ofrecen una densidad energética mucho mayor que los ánodos de grafito utilizados en las baterías actuales. Los electrolitos poliméricos en gel también ofrecen una mayor estabilidad interfacial que sus homólogos líquidos.
Electrolitos poliméricos sólidos frente a gel: Una comparación cara a cara
La elección entre un electrolito polimérico sólido o en gel implica un compromiso entre seguridad, rendimiento y facilidad de fabricación.
| Característica | Electrolitos poliméricos sólidos (SPE) | Electrolitos poliméricos en gel (GPE) |
|---|---|---|
| Seguridad | Máximo (sin líquido inflamable) | Alta (fugas reducidas) |
| Conductividad iónica | Más bajo a temperatura ambiente | Más alto |
| Densidad energética | Potencialmente muy alto | Alta |
| Supresión de dendritas | Excelente | Bien |
| Fabricación | Más complejo | Más maduro |
Ventajas de los electrolitos poliméricos sólidos (SPE)
- Seguridad sin igual: La ausencia de un componente líquido convierte a los SPE en la opción electrolítica más segura.
- Potencial de alta densidad energética: La posibilidad de utilizar ánodos de metal de litio podría suponer un salto significativo en la densidad energética.
- Amplia ventana electroquímica: Los SPE son estables a voltajes más altos, lo que permite el uso de materiales de cátodo de alto voltaje.
Ventajas de los electrolitos poliméricos en gel (GPE)
- Conductividad iónica superior: Los GPE ofrecen un muy necesario aumento de la conductividad, lo que permite una carga más rápida.
- Mejor contacto entre electrodos: Su naturaleza gelatinosa garantiza un contacto íntimo con los electrodos, reduciendo la resistencia.
- Más fácil de fabricar: Los procesos de fabricación de las GPE están más consolidados y se asemejan más a los de las pilas convencionales.
El objetivo último de muchos investigadores es desarrollar un electrolito polimérico sólido que combine la seguridad de un SPE con la alta conductividad iónica de un GPE.
Aplicaciones
El desarrollo de electrolitos poliméricos sólidos y en gel no es sólo un ejercicio académico; tiene el potencial de transformar numerosas industrias.
Revolucionar los vehículos eléctricos
Para la adopción generalizada de los vehículos eléctricos, las baterías deben ser más seguras, tener mayor autonomía y cargarse más rápido. Los electrolitos poliméricos sólidos y de gel responden a estas tres necesidades. La mayor seguridad podría simplificar el diseño de las baterías y reducir su peso y coste. El potencial de una mayor densidad energética podría dar lugar a vehículos eléctricos capaces de viajar mucho más lejos con una sola carga. A medida que Departamento de Energía de EE.UU. sobre baterías de estado sólido señala, esta tecnología es fundamental para el futuro del transporte.
Mejorar la electrónica de consumo
En el mundo de la electrónica de consumo, la tendencia es siempre hacia dispositivos más pequeños, delgados y ligeros. Los electrolitos poliméricos sólidos pueden permitir el diseño de baterías más compactas y flexibles, abriendo nuevas posibilidades para la tecnología vestible y otros dispositivos portátiles. La mayor seguridad también es un argumento de venta importante para los dispositivos que se llevan cerca del cuerpo.
Almacenamiento de energía en red y aeroespacial
Para el almacenamiento de energía a gran escala en la red eléctrica y en aplicaciones aeroespaciales, la fiabilidad y la seguridad son primordiales. La larga vida cíclica y la robustez de las baterías con electrolitos poliméricos las convierten en candidatas ideales para estos exigentes entornos.
Retos y camino por recorrer
A pesar de lo prometedores que son los electrolitos poliméricos sólidos y en gel, aún quedan importantes obstáculos por superar antes de que se generalicen. Como destaca retos en el desarrollo de baterías de estado sólidoNo se trata de cuestiones triviales.
Entre los principales retos figuran:
- Mejora de la conductividad iónica: Para las SPE, lograr una alta conductividad a temperatura ambiente sin comprometer la seguridad sigue siendo un objetivo clave.
- Reducción de la resistencia interfacial: Garantizar una interfaz estable y de baja resistencia entre el electrolito sólido y los electrodos es crucial para el rendimiento a largo plazo.
- Ampliación de la fabricación: El desarrollo de procesos de fabricación rentables y escalables es esencial para la viabilidad comercial.
Los investigadores están explorando activamente diversas soluciones, como el desarrollo de nuevos materiales poliméricos, la creación de electrolitos compuestos que combinen polímeros con cerámica y diseños innovadores de baterías.
Conclusiones: El amanecer de una nueva era de las baterías
Los electrolitos poliméricos sólidos y en gel representan un cambio fundamental en la evolución de la tecnología de las baterías. Ofrecen un camino claro hacia un futuro en el que nuestras soluciones de almacenamiento de energía no sólo sean más potentes y duraderas, sino también fundamentalmente más seguras. Aunque siguen existiendo retos, el ritmo de la innovación en este campo es rápido. Desde la ampliación de la autonomía de los vehículos eléctricos hasta la creación de nuevos formatos para nuestros dispositivos electrónicos, el impacto de esta tecnología se dejará sentir en toda la sociedad. Estamos realmente en los albores de una nueva era de las baterías, que se construirá sobre los sólidos cimientos de la ciencia de los polímeros.