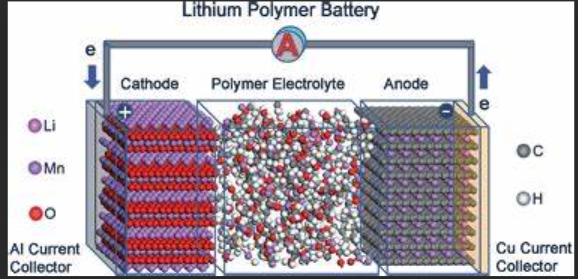
What is the Primary Function of Electrolytes in Lithium Polymer Batteries?
In lithium polymer (LiPo) batteries, electrolytes are crucial in facilitating the movement of lithium ions between the cathode and anode. This movement of ions is what allows the battery to store and release energy. Electrolytes in LiPo batteries typically consist of lithium salts dissolved in a solvent, which enables ionic conduction. Without electrolytes, the essential ion flow needed for charging and discharging would be impossible, rendering the battery non-functional.
How Does Electrolyte Composition Affect Thermal Stability and Flammability?
The composition of the electrolyte in lithium polymer batteries plays a crucial role in determining the battery’s and . When it comes to The composition of the electrolyte in lithium polymer batteries plays a crucial role in determining the battery’s thermal stability and flammability. When it comes to lithium polymer safety, a , a battery’s ability to withstand heat without catching fire is paramount.
-
Liquid electrolytes are commonly used in lithium polymer batteries due to their high ionic conductivity, but they tend to be volatile and highly flammable. In the event of a thermal runaway (a chain reaction of overheating and chemical reactions within the battery), liquid electrolytes can ignite, which increases the risk of fires or explosions. This happens because liquid electrolytes contain solvents, often organic compounds, which are prone to evaporation and combustion at high temperatures. When the battery reaches a critical temperature, these volatile substances can combust or even explode, creating significant safety hazards.
-
Gel electrolytes, on the other hand, are a safer option compared to liquid electrolytes. While they still contain some level of flammable components, the gel structure helps to mitigate some of the risks. Since the electrolyte is less likely to leak, the battery is more resistant to damage from external factors like punctures or temperature fluctuations. Additionally, gel electrolytes offer improved thermal stability because they can endure higher temperatures without breaking down as easily. In some cases, these electrolytes are even designed to function within a wider temperature range, which helps to reduce the chances of overheating.
-
Solid electrolytes are considered the safest option due to their superior thermal stability. They do not contain any solvents, which means they do not carry the same risk of combustion as liquid electrolytes. Solid-state batteries have an electrolyte that is typically made from ceramics or other non-flammable materials, offering excellent heat resistance and stability. Even at high temperatures, these materials are much less likely to degrade, thus significantly reducing the risk of fire or explosion. While solid electrolytes offer superior safety, they are still being developed for widespread commercial use due to higher production costs and more complex manufacturing processes.
What Types of Electrolytes (Liquid, Gel, Solid) Are Safer and Why?
Lithium polymer batteries utilize different electrolyte types depending on the desired performance, safety, and cost factors. The choice of electrolyte type influences the overall lithium polymer safety, performance, and longevity of the battery.
-
Liquid Electrolytes: These are the most commonly used type due to their high ionic conductivity, which ensures efficient charge and discharge cycles. However, liquid electrolytes are generally more flammable and prone to leakage compared to other types. When a lithium polymer battery with liquid electrolyte experiences mechanical damage or overheating, the electrolyte can leak out, causing significant safety risks. In addition, the flammable nature of liquid electrolytes means that they are more likely to catch fire or explode in extreme situations. Although liquid electrolytes are cost-effective and widely used in consumer electronics, their safety concerns make them less desirable for high-performance or high-risk applications.
-
Gel Electrolytes: Gel electrolytes offer a compromise between liquid and solid electrolytes, providing better safety while maintaining high ionic conductivity. These electrolytes are semi-solid, meaning they are less likely to leak compared to liquid electrolytes, and they have improved thermal stability. Gel electrolytes are also safer in the event of physical damage since they are less prone to spilling or causing fires when punctured or impacted. Their semi-solid nature helps to stabilize the battery’s internal environment and prevent the spread of hazardous materials, making them a safer alternative for a variety of consumer electronics and other devices.
-
Solid Electrolytes: Solid-state batteries are emerging as the safest and most advanced technology for lithium polymer batteries. Unlike liquid or gel electrolytes, solid electrolytes are non-flammable and offer significantly improved thermal stability. These electrolytes typically use materials such as ceramics, polymers, or sulfide-based compounds, which are chemically stable and resistant to high temperatures. Solid electrolytes not only improve lithium polymer safety by reducing fire and explosion risks, but they also extend battery life and performance. However, the manufacturing process for solid-state batteries is more complex, which currently makes them more expensive to produce. Despite this, their potential for safer and more efficient batteries has sparked interest in their use for high-end applications like electric vehicles, drones, and high-performance electronics.
Does High Voltage or Fast Charging Degrade Electrolytes and Compromise Safety?
Yes, high voltage and fast charging can degrade the electrolyte in lithium polymer batteries and potentially compromise lithium polymer safety. Both high-voltage operation and rapid charging introduce heat and stress into the battery’s internal structure, which directly impacts the electrolyte and its performance.
-
High Voltage: When a lithium polymer battery is charged or discharged at high voltages, it creates more internal heat, which can cause the electrolyte to break down. Overcharging a battery, especially beyond its safe voltage range, can lead to the breakdown of lithium salts in the electrolyte, resulting in reduced ionic conductivity. This degradation of the electrolyte can create chemical imbalances that trigger thermal runaway—a process where the temperature increases uncontrollably, leading to fires or explosions. For this reason, lithium polymer batteries are designed with built-in voltage limits and management systems to ensure that voltage levels stay within safe operating ranges.
-
Fast Charging: Fast charging puts additional strain on the electrolyte by rapidly pushing a high current through the battery. This generates significant heat, which can raise the internal temperature of the electrolyte beyond its safe operating limit. As the temperature increases, the electrolyte becomes more susceptible to chemical breakdown. This process weakens the battery’s structural integrity, making it more vulnerable to swelling, leakage, and even rupture. In extreme cases, fast charging can lead to the formation of dendrites (lithium metal deposits) that short-circuit the battery and increase the risk of fire. For lithium polymer safety, it is crucial to follow recommended charging practices, including using chargers that regulate voltage and current to prevent overheating.
How Do Extreme Temperatures Affect Electrolyte Performance and Safety?
Extreme temperatures—both high and low—can have a significant impact on electrolyte performance and lithium polymer safety.
-
High Temperatures: Excessive heat is one of the primary factors that can lead to electrolyte degradation in lithium polymer batteries. When exposed to high temperatures, the electrolyte can break down, causing the formation of gases, the release of volatile compounds, and potentially catastrophic thermal runaway. The solvents in liquid electrolytes, for example, may evaporate or react chemically when exposed to heat, further weakening the electrolyte’s ability to conduct ions and leading to potential leakage or fire hazards.
-
Low Temperatures: On the opposite end of the spectrum, low temperatures can also have detrimental effects on electrolyte performance. When lithium polymer batteries are exposed to cold conditions, the viscosity of the electrolyte increases, which reduces its ability to conduct ions efficiently. As a result, the battery’s performance can be severely limited, and it may fail to charge properly or provide sufficient power. At extremely low temperatures, the electrolyte can even freeze, causing irreversible damage to the battery’s structure and the loss of its electrochemical properties. Furthermore, the battery’s internal resistance increases in cold environments, leading to reduced energy efficiency and, in some cases, failure to operate altogether.
Can Electrolyte Leaks Cause External Fire Hazards?
Yes, electrolyte leaks can pose significant external fire hazards. Liquid electrolytes, especially those that are highly flammable, can leak from the battery casing if the battery is damaged. If the electrolyte comes into contact with heat or sparks, it can ignite, leading to dangerous fires. This is why battery manufacturers must ensure proper sealing and protection of electrolytes to minimize the risk of leaks.
Conclusion
The composition of the electrolyte in lithium polymer batteries plays a critical role in ensuring lithium polymer safety. By selecting the right type of electrolyte—whether liquid, gel, or solid—manufacturers can greatly improve the safety, thermal stability, and longevity of batteries. As the demand for high-performance batteries increases, it’s crucial to consider these factors to prevent safety issues and enhance user experience.
FAQs
-
What happens if the electrolyte in a lithium polymer battery is damaged?
Damaged electrolytes can cause leakage, leading to poor battery performance or safety risks such as fires or explosions. -
Are solid-state lithium polymer batteries safer than traditional ones?
Yes, solid-state batteries are safer because they use non-flammable electrolytes, reducing the risk of thermal runaway. -
Can a lithium polymer battery be safely charged in extreme temperatures?
Charging in extreme temperatures is risky, as it can degrade the electrolyte and reduce safety. -
How can you tell if a lithium polymer battery is leaking?
Visible signs of leakage, such as swelling or an unusual smell, are common indicators of a leak in the electrolyte. -
Do all lithium polymer batteries have the same type of electrolyte?
No, lithium polymer batteries can use liquid, gel, or solid electrolytes, each with its advantages and risks.
Landazzle Custom Battery Solutions
At Lan Dazzle, we specialize in producing high-quality, custom lithium polymer batteries that meet the highest safety standards. Whether you need batteries for drones, robots, or consumer electronics, our advanced electrolyte compositions ensure superior lithium polymer safety and performance. Visit www.landazzle.com to learn more about our products and solutions.