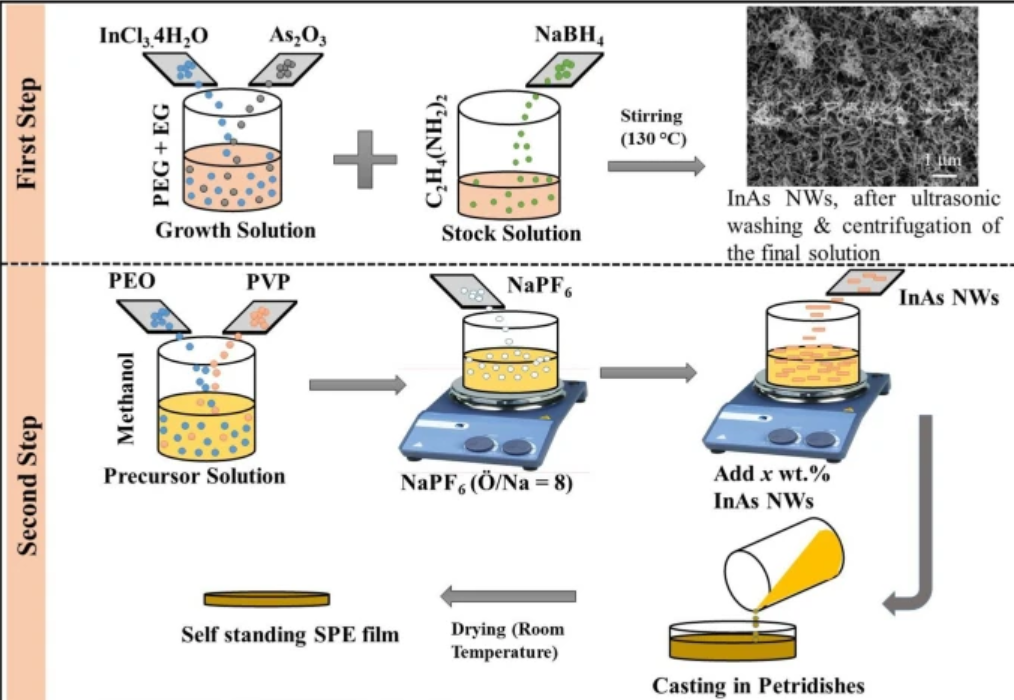
The relentless march of technology demands ever-improving energy storage solutions. From powering the next generation of electric vehicles (EVs) to enabling smaller, longer-lasting consumer electronics and stabilizing our power grids, the need for safer, more powerful, and more flexible batteries has never been greater. For decades, the workhorse of the rechargeable battery world has been the lithium-ion battery with a liquid electrolyte. However, the inherent risks of these liquid electrolytes, such as flammability and leakage, have spurred a global race to develop a superior alternative. Enter the polymer electrolyte, a key enabling technology for the long-awaited era of all-solid-state batteries.
This article delves into the fascinating world of polymer electrolyte manufacturing, offering a comprehensive overview of how these cutting-edge materials are created. We will explore the most common fabrication techniques, from the versatile solution casting method to advanced approaches like 3D printing. By understanding how polymer electrolytes are made, we can appreciate the ingenuity and precision required to build the batteries of the future.
What Exactly Are Polymer Electrolytes?
At its core, a polymer electrolyte is a solid or gel-like material that conducts ions, much like the liquid electrolyte in a conventional battery. It is a composite material, carefully engineered from several key components:
- The Polymer Host: This is the structural backbone of the electrolyte, providing mechanical strength and flexibility. Common polymers used for this purpose include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), polymethyl methacrylate (PMMA), and polyacrylonitrile (PAN). The choice of polymer is critical, as it influences the electrolyte’s thermal stability, mechanical properties, and ionic conductivity.
- The Salt: Dissolved within the polymer host is a salt, typically a lithium salt for lithium-ion batteries, which provides the mobile ions (in this case, lithium ions) that shuttle charge between the battery’s anode and cathode during charging and discharging. Common salts include lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), lithium perchlorate (LiClO₄), and lithium hexafluorophosphate (LiPF₆).
- Additives and Fillers: To further enhance their properties, polymer electrolytes often incorporate other materials. Plasticizers, such as ethylene carbonate (EC) and propylene carbonate (PC), can be added to increase the amorphous phase of the polymer, thereby improving ionic conductivity. Ceramic fillers, like alumina (Al₂O₃), titania (TiO₂), and silica (SiO₂), can be introduced to boost mechanical strength, thermal stability, and suppress the growth of lithium dendrites – a major cause of battery failure.
These components can be combined to create three main types of polymer electrolytes:
- Solid Polymer Electrolytes (SPEs): These are completely solid materials, consisting of a polymer host and a salt. They offer the highest level of safety but have historically suffered from lower ionic conductivity at room temperature.
- Gel Polymer Electrolytes (GPEs): GPEs are a hybrid of solid and liquid electrolytes. They consist of a polymer matrix that traps a significant amount of liquid electrolyte. This results in much higher ionic conductivity than SPEs, while still offering improved safety and form factor flexibility compared to purely liquid electrolytes.
- Composite Polymer Electrolytes (CPEs): These are SPEs or GPEs that have been enhanced with the addition of ceramic or other inert fillers. The fillers can improve a wide range of properties, making CPEs a promising avenue of research.
Common Methods for Making Polymer Electrolytes
The creation of a polymer electrolyte is a meticulous process, with several well-established methods used in both research and industrial settings.
Solution Casting: The Versatile and Widely Used Technique
Solution casting is arguably the most common and straightforward method for preparing polymer electrolyte films, especially in a laboratory environment. The process can be broken down into three simple steps:
- Dissolution: The polymer and the salt (and any other additives) are dissolved in a suitable solvent to form a homogenous solution, often referred to as a “slurry.” The choice of solvent is crucial; it must be able to dissolve all the components without causing any unwanted chemical reactions. Common solvents include acetonitrile, N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO). The mixture is typically stirred for several hours to ensure complete dissolution and uniformity.
- Casting: The resulting viscous solution is poured onto a flat, smooth substrate, such as a glass plate or a Teflon dish. A doctor blade is often used to spread the solution into a thin film of uniform thickness. The thickness of the final membrane can be controlled by adjusting the viscosity of the solution and the height of the doctor blade.
- Evaporation: The cast film is then left in a controlled environment, such as an oven or a vacuum chamber, to allow the solvent to slowly evaporate. This leaves behind a freestanding, solid or gel-like polymer electrolyte membrane.
While simple and versatile, solution casting has its drawbacks. The use of volatile and often toxic organic solvents raises environmental and safety concerns. Additionally, removing all traces of the solvent can be challenging, and any residual solvent can negatively impact the battery’s performance.
Hot-Pressing: A Solvent-Free Approach
To address the limitations of solution casting, the hot-pressing method offers a solvent-free alternative. This technique is particularly well-suited for industrial-scale production. The process involves:
- Mixing: The polymer, salt, and any fillers are thoroughly mixed in their powder form. This can be done using a ball mill or a similar mixing apparatus to ensure a homogenous blend.
- Heating and Pressing: The powder mixture is placed in a mold and heated to a temperature above the polymer’s melting or glass transition temperature. Simultaneously, a high pressure is applied, causing the polymer to soften and flow, encapsulating the salt and fillers.
- Cooling: The mold is then cooled while maintaining the pressure, resulting in a dense, solid polymer electrolyte film.
The hot-pressing method is environmentally friendly as it eliminates the need for solvents. The resulting membranes are often mechanically robust and have good interfacial contact when pressed directly onto electrodes. However, achieving a completely homogenous mixture of the components can be challenging, and the high temperatures and pressures required can sometimes degrade the polymer or salt.
Electrospinning: Creating Nanofibrous Membranes
Electrospinning is a fascinating technique that uses electrostatic forces to produce extremely fine polymer fibers, typically on the order of nanometers in diameter. These nanofibers can be collected as a non-woven mat, which can serve as a highly porous polymer electrolyte membrane. The process works as follows:
- Solution Preparation: A polymer solution with the desired salt and additives is prepared, similar to the solution casting method.
- Ejection: The solution is loaded into a syringe with a metallic needle. A high voltage is applied to the needle, which creates a strong electric field between the needle tip and a grounded collector screen.
- Fiber Formation: When the electrostatic forces overcome the surface tension of the polymer solution, a jet of the solution is ejected from the needle. The solvent rapidly evaporates as the jet travels towards the collector, and the jet is stretched and thinned into a continuous nanofiber.
- Kolekcja: The solid nanofibers are collected on the screen as a porous, web-like membrane.
The key advantage of electrospun polymer electrolytes is their incredibly high surface area and interconnected porosity. This structure can be filled with a liquid electrolyte to create a GPE with exceptionally high ionic conductivity, as the liquid has continuous pathways for ion transport.
In-Situ Polymerization: Building the Electrolyte Inside the Battery
The in-situ polymerization method takes a different approach: instead of fabricating the electrolyte separately and then assembling it into a battery, the electrolyte is created directly within the battery cell. This is typically done by:
- Precursor Injection: A liquid precursor mixture, containing monomers (the building blocks of polymers), a salt, and a polymerization initiator, is injected into the battery cell, filling the space between the anode and the cathode.
- Polymerization: The battery is then exposed to heat or ultraviolet (UV) light, which triggers the polymerization process. The monomers link together to form a solid or gel polymer electrolyte that is intimately integrated with the electrodes.
This method offers the significant advantage of creating a seamless interface between the electrolyte and the electrodes, which minimizes interfacial resistance and improves battery performance. It also allows for the creation of batteries in a variety of shapes and sizes.
Advanced Polymer Electrolyte Manufacturing
Research in polymer electrolyte fabrication is constantly evolving, with new and innovative techniques emerging.
Phase Inversion: Crafting Porous Structures
Phase inversion is a process where a polymer solution is induced to separate into a polymer-rich phase and a polymer-poor phase. By carefully controlling this separation, it is possible to create highly porous membranes. One common way to induce phase inversion is to immerse a cast polymer film in a non-solvent bath. The solvent in the film exchanges with the non-solvent, causing the polymer to precipitate and form a porous structure. These porous membranes can then be filled with a liquid electrolyte to form a GPE.
3D Printing: The Future of Customized Electrolytes
3D printing, or additive manufacturing, is poised to revolutionize the design and fabrication of batteries. By using a special “ink” made from a polymer electrolyte precursor, it is possible to print electrolytes with complex, three-dimensional architectures. This could enable the creation of microbatteries for tiny electronic devices, or batteries with interdigitated electrodes for high power applications. While still in the early stages of development, 3D printing offers unprecedented design freedom for the batteries of the future.
Quality Control: Characterizing Polymer Electrolytes
Once a polymer electrolyte has been fabricated, it must undergo a series of rigorous tests to ensure it meets the demanding requirements of a battery. Key properties that are evaluated include:
- Ionic Conductivity: This is a measure of how well the electrolyte conducts ions. A high ionic conductivity is essential for a battery that can deliver high power.
- Electrochemical Stability Window: This is the range of voltages over which the electrolyte remains stable without decomposing. A wide stability window is necessary for high-voltage batteries.
- Mechanical Strength: The electrolyte must be strong enough to withstand the stresses of battery assembly and operation, and to suppress the growth of lithium dendrites.
- Thermal Stability: The electrolyte must be able to operate safely over a wide range of temperatures.
Conclusion: The Road Ahead for Polymer Electrolyte Manufacturing
The manufacturing of polymer electrolytes is a dynamic and rapidly advancing field. While solution casting remains a popular and versatile technique, methods like hot-pressing, electrospinning, and in-situ polymerization are becoming increasingly important for creating high-performance, safe, and reliable solid-state batteries. The journey towards the widespread adoption of polymer electrolytes is not without its challenges. Scaling up production, reducing costs, and further improving performance are all key areas of ongoing research. However, with continued innovation in manufacturing, polymer electrolytes are set to play a pivotal role in the clean energy transition, powering a more sustainable and electrified future.